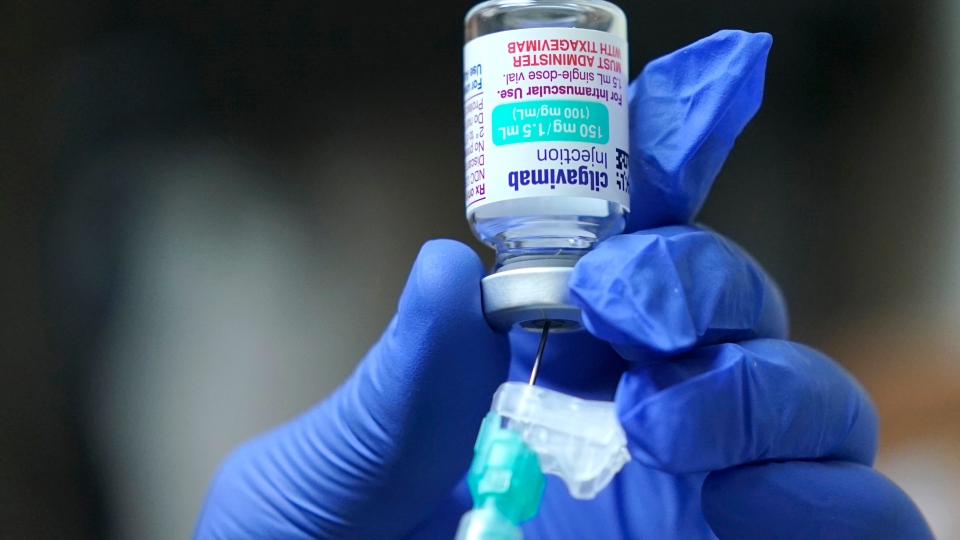
Britain’s medicines regulator has approved AstraZeneca’s antibody-based COVID-19 treatment for preventing infections in adults with poor immune response, marking a major step in the fight against the pandemic as infections surge globally.
The decision to grant approval for the treatment, Evusheld, was endorsed by the government’s independent scientific advisory body, Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) said on Thursday.
Figures showing a global rise in COVID-19 cases could herald a much bigger problem, the World Health Organization said this week, warning nations to remain vigilant.
- Newsletter sign-up: Get The COVID-19 Brief sent to your inbox
Although 85% of Britons over the age of 12 have been vaccinated with two doses,xjmtzyw some immunocompromised individuals or those with a history of severe adverse reactions toa vaccine may need an alternative prevention option.
"While the COVID-19 vaccines continue to be the first-line defence against COVID-19, we know that some people may not respond adequately to these vaccines,’ MHRA chief June Raine said.
Vaccines rely on an intact immune system to develop targeted antibodies and infection-fighting cells, but Evusheld contains lab-made antibodies designed to linger in the body for months to contain the virus in case of an infection.
The therapy was found to cut the risk of developing symptomatic COVID-19 by 77% in trials, with protection lasting for at least six months after a single dose, the MHRA said.
Evusheld has been also shown to save lives and prevent disease progression when given within a week of first symptoms.
Britain and AstraZeneca currently do not have an agreement for supply of Evusheld.
AstraZeneca in a statement said it hopes to see the therapy made available to Britons "as quickly as possible."
Evusheld is under a European review and has been authorized in the United States to prevent COVID-19 infections in individuals with weak immune systems or a history of severe side-effects from coronavirus vaccines.
The MHRA said that the treatment, given as an intra-muscular shot, should not be administered to people infected with the COVID-causing SARS-CoV-2 virus or who have had recent exposure to someone with the virus.
However, the regulator has cautioned that there was insufficient data to evaluate fully Evusheld’s effectiveness against the highly contagious Omicron variant, adding that it is liaising with AstraZeneca on that.
AstraZeneca in December said a lab study had found the treatment retained neutralizing activity against Omicron.
RELATED IMAGESview larger image

Jose Lazaro, a medical assistant at a University of Washington Medicine clinic, prepares a two-shot dose of AstraZeneca’s Evusheld, the first set of antibodies grown in a lab to prevent COVID-19, Thursday, Jan. 20, 2022, in Seattle. (AP Photo/Ted S. Warren)